
The UKHSA's International Health Regulations Strengthening Project (IHR-SP) conducted a first of its kind workshop on 8-11 July 2024 aimed at building the capacity of EPHI to test for diphtheria and pertussis. This was co-delivered in partnership with EPHI. The training was facilitated by IHR-SP subject matter experts, Aodhan Breathnach and Jumoke Sule and the in-country laboratory technical advisor, Hiwot Amare Hailu. The training was also co-facilitated by EPHI laboratory experts Etsehiwot Adamu and Meseret Assefa, who had previously participated in a similar WHO and UKHSA-led workshop in Cyprus demonstrating sustainability and transferability of skills gained through IHR-SP training.
The WHO African Region is dealing with a resurgence of diphtheria, a rare and deadly disease, with five active outbreaks ongoing in Guinea, Mauritania, Niger, Nigeria and South Africa. Ethiopia has been identified as one of the most vulnerable countries raising concerns about potential disease transmission. It is also affected by war, conflict, and other social disruption, which leads to the displacement of populations, overcrowding, and breakdown in vaccination programmes. Improving diphtheria laboratory diagnostic capacity, especially at the national reference laboratory, is crucial for reliable outbreak confirmation as part of the country’s emergency preparedness plan. EPHI acknowledged this and requested support from IHR-SP to strengthen national detection capacity in diphtheria and pertussis. This will help by enabling prompt implementation of appropriate prevention and control measures.
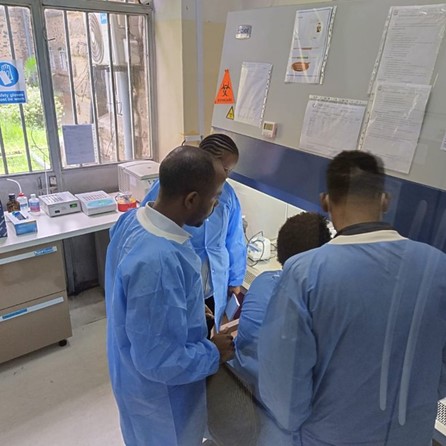
Figure 1. Trainees in the laboratory during practical sessions
Participants were drawn from the EPHI National Bacteriology Reference Laboratory and Surveillance Team. There were presentations on the global and regional overview of diphtheria and pertussis, followed by laboratory practical demonstrations on how to extract nucleic acid from samples and amplify DNA using Rotor gene polymerase chain reaction (PCR) machine.
At the end of the practical sessions, group discussions were held to identify the next steps. Participants identified the following as priorities:
- sample collection, handling, and transportation as well as reporting system training
- verification of the assays, strengthened quality management and standard operating procedures
- advocacy for establishment of a resilient supply chain for the procurement of reagents to ensure availability of molecular diagnosis of diphtheria and pertussis
- consideration of the introduction of routine culture as well as PCR
- identifying sentinel sites for surveillance for diphtheria and pertussis
The feedback from the training was positive; all participants strongly agreed that the workshop was relevant to what they do and indicated that they would apply the skills learned in their jobs. Participants also either strongly agreed or agreed that the objectives of the training were clear, that the training materials were easy to understand, and that they have the skills on proper use of pipetting, extracting bacterial DNA and preparing master mix reagents.
Hiwot Hailu, IHR-SP’s Technical Laboratory Advisor for Ethiopia said: “As parts of the African region face outbreaks of diphtheria, it is timely for IHR-SP to partner with EPHI, support Ethiopia’s emergency preparedness plan and strengthen national diagnostic capacity for diphtheria and pertussis. Delivering this first of its kind training to key National Reference Laboratory staff will enable prompt confirmation of suspected outbreaks and implementation of necessary prevention and control measures. IHR-SP will continue to work with EPHI and the National Reference Laboratory to strengthen capacity and capability in identified priority areas of need.”
 Figure 2. Participants and facilitators at the end of the workshop
Figure 2. Participants and facilitators at the end of the workshop