Strengthening Laboratory Management Towards Accreditation (SLMTA) is an approach to laboratory quality management systems (QMS) designed to produce measurable improvement and prepare laboratories for accreditation based on international clinical laboratory standards. The process of achieving this accreditation can be labour-intensive, complex, and expensive. One of the specific innovative tasks recommended under the SLMTA programme is to implement the WHO AFRO Stepwise Laboratory Quality Improvement Process Towards Accreditation (SLIPTA) framework.
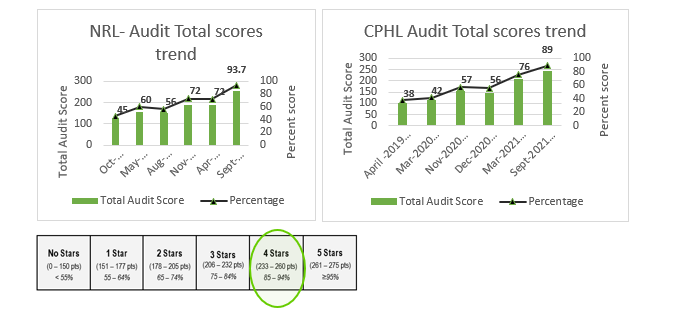
The UKHSA IHR Strengthening project has been supporting the Nigeria Centre for Disease Control (NCDC) National Reference Laboratories (NRL) and Central Public Health Laboratories (CPHL) in governance strengthening and Laboratory Quality Management Systems (QMS) towards ISO15189 accreditation through SLMTA since 2018. In 2018 and 2021, UKHSA assessed both NRL and CPHL laboratories to determine the level of their QMS using the SLIPTA checklist. Although an improvement was observed for NRL in the audit score from zero star (45%) in 2018 to 2 stars (72%) in 2021, and an improvement for CPHL from zero star to 3 stars, some deficiencies were still noted in the implementation of QMS, indicating the need to further strengthen NCDC laboratory QMS. Areas of observation and noted gaps were targeted by UKHSA in-country technical advisers and UK-based subject matter experts supporting remotely. The focus was on reviewing and completing outstanding SOPs and Manuals. Training of laboratory quality teams (QT) on ISO15189 standards, specifically addressing areas such as internal auditing, laboratory ethics, resolving and documentation of non-conformities (NC) and tracking and documentation of Key performance indicators (KPI).
Two key training workshops were delivered in June and July 2021 designed to target the nonconformities raised at the previous audit of March/ April 2021. This resulted in the resolution of >95% of all the NC. 5% of the NC remain unresolved currently being addressed by the management team.
With support from the IHR Strengthening project a follow-on audit performed in September 2021 showed a fantastic improvement resulting in a WHO-Afro star 4 rating for both laboratories. This is a great achievement and a firm recognition of the excellent work undertaken with support from the IHR Strengthening project particularly in terms of training and continual mentoring.
The UKHSA effort was commended by the outgoing Director General of NCDC, Dr. Chikwe Ihekweazu:
“Before 2016, we did not have a reference laboratory with the capacity to detect and diagnose many infectious diseases. In the last five years, the work that each member of staff in the laboratories have put in day in, and day out has contributed to strengthening our national health security and in turn, global health security.
I am extremely proud of the work that you have done, and you can be proud. The transformation that Mrs Mba, Mrs Babatunde, Mr Ekoh as well as Mrs Okoi have driven is absolutely phenomenal. Together with your teams and the incredible support from our partners particularly Bunmi in PHE (now UKHSA) and McPaul in US CDC in this regard will never be forgotten. “
In response, Bunmi Negedu-momoh, (UKHSA Laboratory Technical Advisor to the DG, NCDC) in celebration of this achievement declared:
’It was much relief on my side to move the lab from uncertainty to absolute level – star 4, a lot of sleepless nights and doggedness too. Thanks to my UKHSA colleagues, Colin, Bee, and Bisi for their indivisible support for me to strengthen the lab’
The laboratory leadership and members of the QT recognise the input of the UKHSA IHR Strengthening Project as they continue to work towards WHO-Afro lab rating of 5 stars.